Health ministry recalls blood pressure drug over labeling error
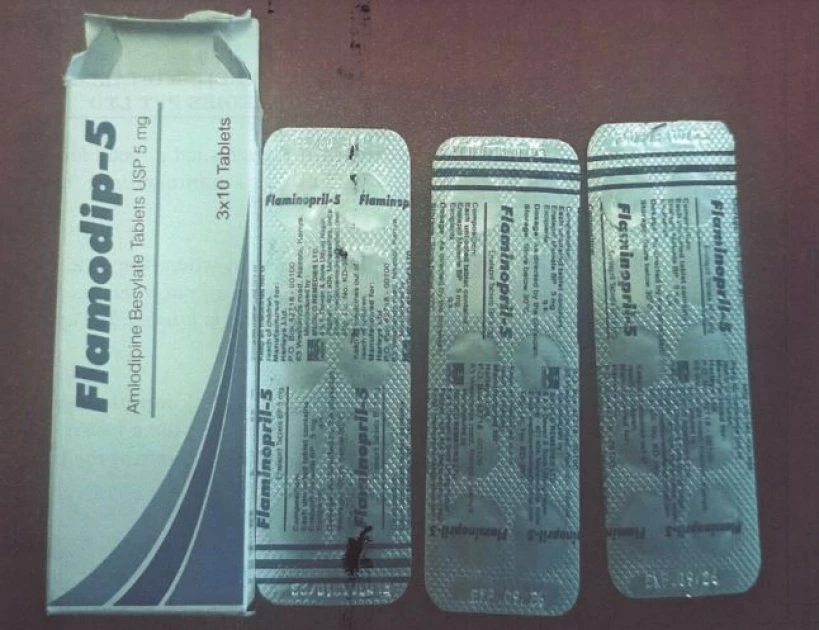
Flamodip (Amlodipine) tablets.

Audio By Vocalize
The Ministry of Health, through the Pharmacy
and Poisons Board (PPB), has recalled a batch of 5mg Flamodip (Amlodipine)
tablets used to treat conditions such as high blood pressure.
In a statement on Friday, PPB CEO Dr. Fred
Siyoi highlighted that the recall, attributed to a labeling error, affects
Batch No. FLD303 of the cardiovascular drug manufactured by Medico Remedies Pvt
Ltd.
"The recall has been initiated due to a
labeling error, where the product's label does not accurately reflect its
contents. The secondary packaging is labeled as Flamodip-5 (Amlodipine), while
the primary packaging is labeled as Flaminopril-5 (Enalapril)," he said.
Dr. Siyoi further instructed all
pharmaceutical outlets, healthcare facilities and professionals to immediately
halt the distribution, sale, and use of the recalled batch.
"The Board advises all pharmaceutical
outlets, healthcare facilities, healthcare professionals and members of the
public to STOP further distribution, sale, issuing or use of the product batch
and return the product to their nearest healthcare facility or respective
suppliers," he said.
Additionally, the PPB boss urged members of the public to report any suspected cases of sub-standard medicines or adverse drug reactions to the board through its official website, email, mobile number, mPvERs mobile application and USSD code *271#.

Leave a Comment