Gov’t cautions Kenyans against counterfeit diabetes drug after Interpol alert
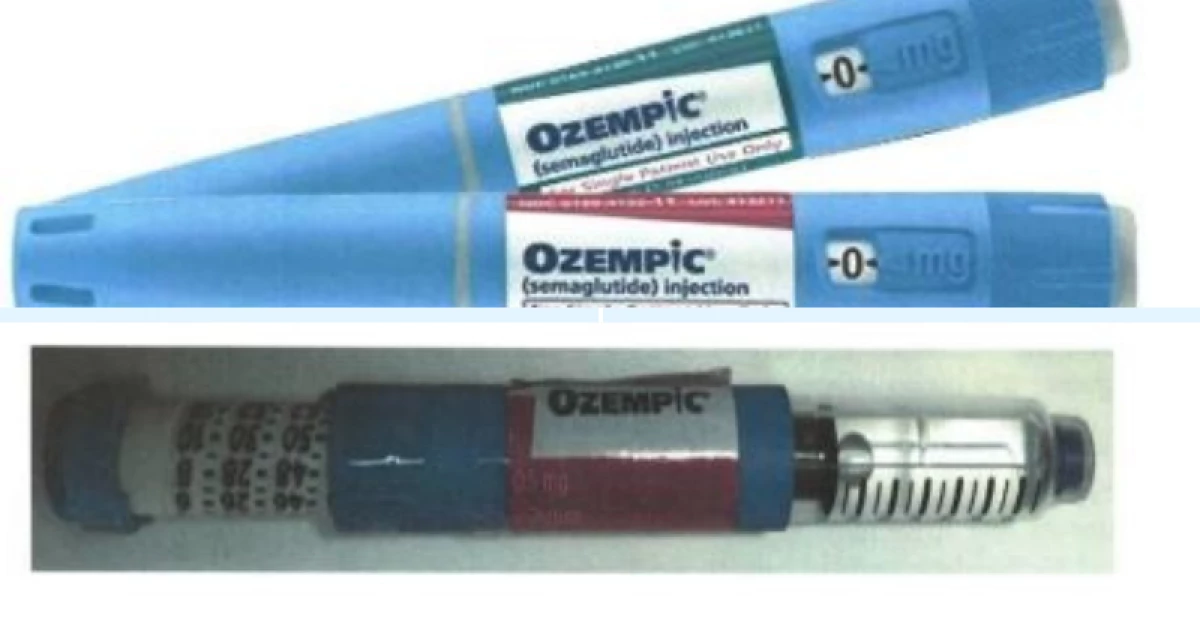
Genuine Ozempic Pens (top) and a falsified Ozempic Pen. PHOTO/COURTESY: PPB

Audio By Vocalize
The Pharmacy and Poisons Board (PPB) has
raised alarm over the counterfeiting of Ozempic Pens, which are injecting
prescription drugs prescribed for adults with type 2 diabetes.
In a statement released on Thursday, PPB CEO
Dr. Fred Siyoi cited an Interpol alert warning about the falsification of
Ozempic Pens.
According to Interpol, Apidra Solostar pens,
which are used to treat both type 1 and type 2 diabetes, are being falsely
re-labelled as Ozempic Pens in various markets.
"The Pharmacy and Poisons Board wishes
to draw the attention of the public to an alert received from the Interpol concerning
the falsification of Ozempic Pens (Semaglutide) where Apidra Solostar pens
(glulisine) used to treat both type 1 and type 2 diabetes has been falsely
relabelled as Ozempic (Semaglutide) Pens," said Siyoi.
Furthermore, Dr, Siyoi emphasized that
Ozempic Pens are currently not authorized, by the PPB, for sale in the Kenyan
market.
"Therefore, any product being marketed
as Ozempic Pens is illegally in the market and the Board cannot ascertain their
safety, quality and effectiveness," he noted.
Dr. Siyoi added that PPB has initiated a
rapid response and heightened surveillance to verify whether the falsified
Ozempic Pens are presently circulating in the Kenyan market.
"The Board cautions the public and
healthcare professionals against trading, distribution, wholesaling, retailing,
issuing, dispensing, use or administration to patients of the falsified Ozempic
(Semaglutide) Pens, as such actions are illegal and jeopardise public health
and safety," he stated.
He further urged members of the public and
healthcare professionals to immediately share any information regarding falsified
Ozempic pens with PPB.

Leave a Comment